Introduction
UMCES Faculty Jeremy Testa and Ming Li are collaborating on a research project, which aims to determine if adding alkaline materials to wastewater treatment discharge has two environmental benefits:
- Bolstering the carbon consuming capacity of the ocean to help fight climate change
- Reducing the harmful effects of ocean acidification
This project will evaluate the feasibility and cost of these approaches, as well as develop a protocol for measuring and monitoring carbon removal.
________________________________________________________________________________________________________________________________________________________________
Pilot Study Starting October 2024
We are excited to begin our first trial at the Hampton Roads Sanitation District VIP facility in Norfolk, Virginia. The trial is planned to run from October 22 to November 6 and involves a small-scale experiment to add lime during various stages of the sewage treatment process.
One part of the trial will add lime addition at the outfall, and we will measure how much more carbon dioxide the Elizabeth River takes up.
A second part of the trial adds lime in the middle of the treatment process, and we will measure how much less carbon dioxide the facility emits.
Stay tuned for updates on the trial.

HOW DOES IT WORK?
"Alkalinity" describes water’s ability to resist changes in pH with acid addition. When water is higher in alkalinity, changes in chemistry convert the acidic dissolved carbon dioxide in the water into bicarbonate, a form of inorganic carbon similar to baking soda dissolved in water. When alkalinity is added to ocean water, there is a reduction in dissolved carbon dioxide in the water, meaning the ocean can absorb more carbon dioxide from the atmosphere. These chemical changes also work to make the water less acidic.
HOW IS ALKALINITY ADDED TO THE OCEAN VIA WASTEWATER?
There are many forms of alkalinity that can be added to the ocean. Common forms include magnesium hydroxide and calcium oxide/hydroxide. These types of alkalinity are already commonly added to wastewater because the processes that wastewater treatment plants use to remove nutrients require extra alkalinity. Theoretically, sodium hydroxide (which can be used as a detergent) can also be added to the ocean, but because it has ecological impacts, it will not be studied in this trial.
WHY IS THIS RESEARCH IMPORTANT?
This research is part of a global effort to find new ways to remove carbon dioxide from the atmosphere and combat climate change. Manipulating wastewater treatment plant procedures and discharge to enhance carbon removal is a practical way to do this because of the current readiness of infrastructure in these plants to deliver alkalinity to the coastal ocean. Many wastewater facilities already add alkalinity to enhance treatment performance, permits to allow alkalinity discharge already exist, and there are several known technologies that can increase alkalinity in wastewater streams.
Despite the existence of this "shovel-ready" carbon removal technology, there is still uncertainty regarding its efficiency, and if it can be scaled up to have a global impact. These uncertainties center around cost, the efficacy of the process at a scale that can meaningfully impact coastal waters, and the environmental and biological impacts of various alkalinity-rich materials.
This research will help quantify the feasibility of this approach and evaluate carbon removal and ocean acidification mitigation via alkalinity enhancement at a wastewater treatment plant in the Chesapeake Bay.
HOW WILL THIS RESEARCH BE CONDUCTED?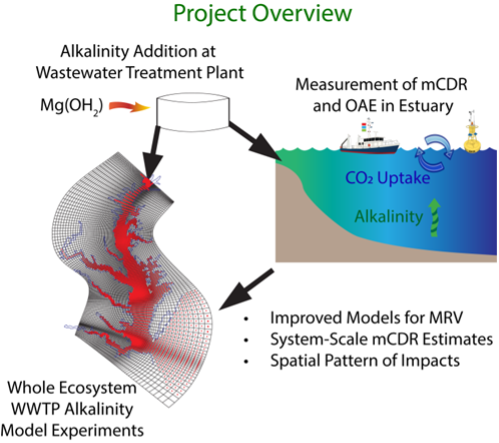
This project will add alkalinity at a single wastewater treatment plant in the Hampton Roads Sanitation District in Virginia. Watch a movie of this facility and its nutrient removal technology by following this link.
In the first year, a one-week test will ensure the safety and rigor of the dosing method. The team will closely monitor and control alkalinity dosing rates within the facility and then concurrently monitor the receiving tidal waters for carbon removal and environmental impacts.
During the second year, the researchers will perform a four-week test. This longer test will help identify other natural factors that modify the potential effect of carbon removal, such as the phase of the tide, the amount of algal growth in the water, and weather. During both tests, the team will monitor oyster growth and other environmental parameters like pH (a measure of the acidity of the water), total suspended solids, nutrient concentrations, metals and carbon chemistry variables. The results of these two tests will inform an ocean model to better understand the benefits and impacts of a potential scaled-up version of these small field tests.
Testa and Li are also collaborating with Wei-Jun Cai, University of Delaware; Charles Bott, Hampton Roads Sanitation District, and William Burt of Planetary.
LOOKING AHEAD
The results of the team's research will be posted to this page in late 2024, or early 2025; however, there are many resources on Ocean Alkalinity Enhancement for you to explore below, which are developed by public institutions, private companies and foundations:

